Pollutants in the atmosphere mix with water in the air to form acid rain. Sulphur dioxide (SO2) and nitrogen oxides (NOx) are the two main pollutants that form acid rain. SO2 reacts with water in the air to form sulphurous acid (H2SO3), and then with oxygen to form sulphuric acid (H2SO4). NOx reacts with water to form nitric acid (HNO3).
Acid rain causes damage to land and water ecosystems, as well as to human-made materials and structures such as buildings and statues. Human health impacts appear to be mostly attributable to SO2 and to the formation of fine particulate and ozone (the main components of smog) from SO2 and NOx.
Damage to land and water ecosystems occurs when the land, water or plants like trees and crops cannot neutralize the acid being deposited by the rain. High levels of acidity can destroy life in our lakes and rivers and reduce forest growth. Nova Scotia has low tolerance for acid precipitation because of the low buffering capacity and neutralization abilities of water and land ecosystems in most of the province, especially in south-western Nova Scotia.
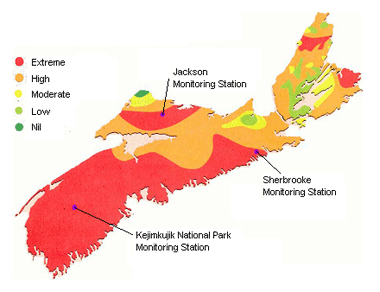
This map shows areas where lakes are extremely sensitive to acidification as red, and areas where lakes are highly sensitive as orange. (Source: The State of the Nova Scotia Environment, July 1998)
Critical load is the amount of acid precipitation that an ecosystem can endure before long-term harmful effects occur. The critical load threshold for lakes in Nova Scotia is 8 kilograms of sulphate per hectare per year (kg/ha/y).
The existing critical loads (kg/ha/y) were developed only for water ecosystems and were defined as the level of wet sulphate deposition that would maintain a pH of 6 in 95% of lakes (1997 Canadian Acid Rain Assessment). (A pH of 6 is a benchmark threshold for sustaining fresh water systems).
Very recently, the science of critical loads and measurements to support them has advanced and critical loads for land ecosystems have been developed. For the first time, this has enabled development of "combined ecosystem (water and land) critical load" maps. These combined ecosystem maps are expressed in different units (eq/ha/yr) than for water ecosystems only (kg/ha/y), because different chemical species and wet and dry deposition must be combined in the calculation.
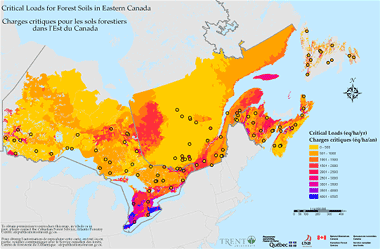
The map above shows the critical loads for forest soils in eastern Canada (Ontario, Quebec, New Brunswick and Nova Scotia). Nova Scotia has among the lowest critical load thresholds in eastern Canada.
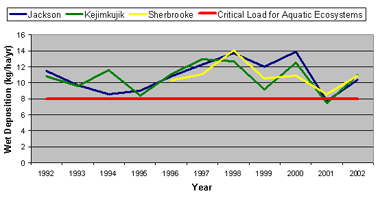
Annual Sea-salt Corrected Sulphate Deposition in Nova Scotia, 1992-2002 - The graph above shows wet sulphate deposition measured at three stations in Nova Scotia against the critical load for lakes in the province. Nova Scotia continues to exceed critical loads for both water and land ecosystems. Our goal is to meet the threshold for critical loads for acid deposition in the province. (Data source: NAtChem database)
In addition to SO2 and NOx pollution from local provincial sources, Nova Scotia receives a great deal of trans-boundary air pollution from other areas in Canada and the United States. The 2004 Canadian Acid Deposition Science Assessment provides recent estimates of the major source regions affecting Nova Scotia ecosystems.
